Purpose
- Clinical benefit of aerosol medication can be compromised when using a pressurized metered dose inhaler (MDI) alone.
- Short delays between actuation and inhalation have been shown to significantly reduce medication delivery (Suggett et al., 2020).
- Even with perfect coordination, much of the aerosol is deposited in the oropharynx, increasing risk for bacterial infection and complications to oral health.
- Such risks can be mitigated through the use of a valved holding chamber (VHC).
Methods
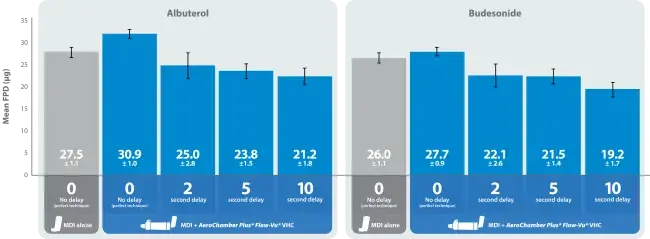
- Airsupra† is a novel MDI that offers a convenient approach to asthma management, combining the fast-acting relief of albuterol with the anti inflammatory effects of budesonide. This laboratory study sought to determine if a VHC could alleviate the loss of Airsupra† drug available when there is a delay between actuation and inhalation.
- Measurements for fine particle dose (FPD, <4.7μm) for the Airsupra† (albuterol 90μg / budesonide 80μg) MDI were made by a cascade impactor at 28.3L/min. Tests with the pMDI alone only included the perfect, but unlikely, condition where there was no-delay between actuation and simulated inhalation. Four sampling conditions were evaluated with AeroChamber Plus® Flow-Vu® VHC: immediate collection with no delay between actuation and inhalation, simulating perfect coordination; and collection after a 2, 5 and 10 second delay, simulating an uncoordinated patient use scenario. Five tests were completed for each condition.
Results